2-cyanopyridine derivatives enable N-terminal cysteine bioconjugation and peptide bond cleavage of glutathione under aqueous and mild conditions†
Abstract
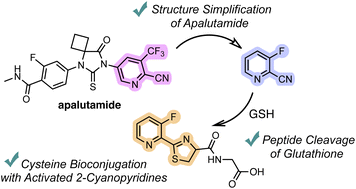
Inspired by the chemical reactivity of apalutamide, we have developed an efficient method for N-terminal cysteine bioconjugation with 2-cyanopyridine derivatives. Systematic investigations of various 2-cyanopyridines revealed that 2-cyanopyridines with electron-withdrawing groups react efficiently with cysteine under aqueous and mild conditions. Moreover, the highly reactive 2-cyanopyridines enable the peptide bond cleavage of glutathione. The utility of our method is demonstrated by its application to the cysteine-selective chemical modification of bioactive peptides.


 Please wait while we load your content...
Please wait while we load your content...