Iodine-PEG as a unique combination for the metal-free synthesis of flavonoids through iodonium-triiodide ion-pair complexation†
Abstract
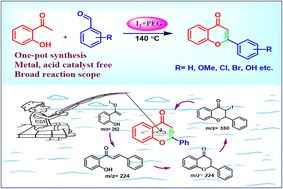
An efficient metal-free single-step protocol has been developed for the direct synthesis of flavones from 2-hydroxyacetophenone and substituted benzaldehydes. This chemical transformation is exclusively promoted by the iodonium-triiodide ion couple formed through iodine and PEG-400 complexation. The triiodide anion not only helps in the abstraction of a proton from the acetophenone but also promotes the cyclization of intermediate chalcone to the corresponding flavones. The flavones were obtained in very high yields without using any toxic metal catalysts or harsh reaction conditions. The reaction mechanism was established through a series of test reactions and entrapping of reaction intermediates. The developed protocol provides direct access to flavones in high yields under milder reaction conditions with great substrate compatibility, including hydroxylated derivatives.


 Please wait while we load your content...
Please wait while we load your content...