Design, synthesis and biological evaluation of indole-2-carboxylic acid derivatives as novel HIV-1 integrase strand transfer inhibitors†
Abstract
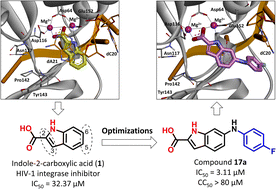
Integrase plays an important role in the life cycle of HIV-1, and integrase strand transfer inhibitors (INSTIs) can effectively impair the viral replication. However, drug resistance mutations have been confirmed to decrease the efficacy of INSTI during the antiviral therapy. Herein, indole-2-carboxylic acid (1) was found to inhibit the strand transfer of integrase, and the indole nucleus of compound 1 was observed to chelate with two Mg2+ ions within the active site of integrase. Through optimization of compound 1, a series of indole-2-carboxylic acid derivatives were designed and synthesized, and compound 17a was proved to markedly inhibit the effect of integrase, with IC50 value of 3.11 μM. Binding mode analysis of 17a demonstrated that the introduced C6 halogenated benzene ring could effectively bind with the viral DNA (dC20) through π–π stacking interaction. These results indicated that indole-2-carboxylic acid is a promising scaffold for the development of integrase inhibitors.


 Please wait while we load your content...
Please wait while we load your content...