Synthesis of N-glycosyl amides: conformational analysis and evaluation as inhibitors of β-galactosidase from E. coli†
Abstract
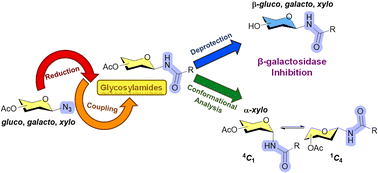
The synthesis of N-glycosyl amides typically involves the use of glycosyl amines as direct precursors, resulting in low yields due to hydrolysis and the loss of stereocontrol through anomerization processes. In this study, a sequential synthesis of N-glycosyl amides is proposed, employing glycosyl amines as intermediates obtained from glycosyl azides. Derivatives with gluco, galacto, or xylo configurations were synthesized. Hexose derivatives were obtained under stereocontrol to give only the β anomer, while the xylo derivatives provided a mixture of α and β anomers. Conformational analysis revealed that all β anomers adopted the 4C1 conformation, while α anomers were found in the 1C4 chair as the major conformer. After de-O-acetylation, the derivatives containing a galactose unit were evaluated as inhibitors of β-galactosidase from E. coli and were found to be moderate inhibitors.


 Please wait while we load your content...
Please wait while we load your content...