Enantioselective synthesis of α-tetrasubstituted (1-indolizinyl) (diaryl)-methanamines via chiral phosphoric acid catalysis†
Abstract
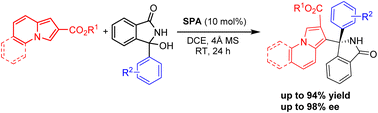
An enantioselective Friedel–Crafts reaction of cyclic α-diaryl N-acyl imines with indolizines catalyzed by a chiral spirocyclic phosphoric acid has been developed. The asymmetric transformation proceeds smoothly to afford α-tetrasubstituted (1-indolizinyl) (diaryl)methanamines in good yields with up to 98% ee under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...