A solid-state electrolyte for electrochemical lithium–sulfur cells†
Abstract
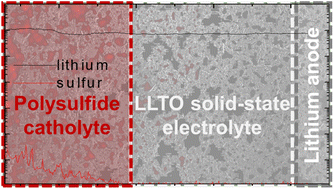
Post-lithium-ion batteries are designed to achieve high energy density and high safety by modifying their active material and cell configuration. In terms of the active material, lithium–sulfur batteries have the highest charge-storage capacity and high active-material utilization because of the use of a conversion-type sulfur cathode, which involves conversion between solid-state sulfur, liquid-state polysulfides, and solid-state sulfides. In terms of the configuration, solid-state batteries ensure high safety by using a solid-state electrolyte in between the two electrodes. Herein, we use a lithium lanthanum titanate (LLTO) solid-state electrolyte in the lithium–sulfur cell with a polysulfide catholyte electrode. The LLTO, which replaces the conventional liquid electrolyte, is a solid-state electrolyte that offers smooth lithium-ion diffusion and prevents the loss of polysulfides, while the highly active polysulfide electrode, which replaces the solid-state sulfur cathode, improves the reaction kinetics and the active-material utilization. The material and electrochemical analyses confirm the stabilized electrodes exhibit long-lasting lithium stripping/plating stability and limited polysulfide diffusion. Moreover, the morphologically and electrochemically smooth interface between the solid-state electrolyte and catholyte enables fast charge transfer in the cell, which demonstrates a high charge-storage capacity of 1429 mA h g−1, high rate performance, and high electrochemical efficiency.


 Please wait while we load your content...
Please wait while we load your content...