Electrochemical remote C(sp3)–H thiocyanation†
Abstract
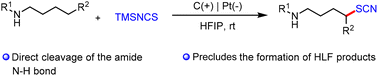
The amidyl radical-mediated 1,5-HAT is a powerful strategy to realize distal C(sp3)–H functionalization. Recently, organic electrosynthesis has provided a sustainable method for the generation of nitrogen-centered radicals. However, current approaches based on electrochemically promoted 1,5-HAT are limited to the Hofmann–Löffler–Freytag (HLF) reaction. We report herein a solution to this challenge by trapping the generated carbocation with TMSNCS to achieve the distal C(sp3)–H thiocyanation. Control experiments and DFT calculations support the radical–polar crossover mechanism. The reaction has a broad substrate scope, including primary, secondary, and tertiary substrates and bioactive molecules.


 Please wait while we load your content...
Please wait while we load your content...