Catalytic asymmetric ring-opening of aminocyclopropanes with oxygen nucleophiles: access to chiral γ-amino acid derivatives†
Abstract
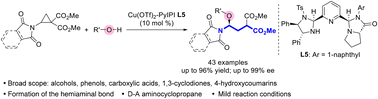
Donor–acceptor aminocyclopropanes containing both amino and carboxyl groups underwent efficient catalytic asymmetric ring-opening reactions with diverse oxygen nucleophiles to afford chiral γ-oxygen-substituted γ-aminobutyric acid derivatives in 60–96% yields and 70–99% ee. With imidazoline-pyrroloimidazolone pyridine as the chiral ligand, a broad range of oxygen nucleophiles, including alcohols, phenols, carboxylic acids, 4-hydroxycoumarins and 1,3-cyclodiones are compatible. The developed method was applied to the derivatization of complex molecules including (−)-myrtenol, stavudine, (S)-naproxen, and (−)-estrone.


 Please wait while we load your content...
Please wait while we load your content...