Transition-metal-catalyzed straightforward synthesis of N-trifluoromethyl indoles from 2-alkynylaryl isothiocyanates or 2-alkynylanilines†
Abstract
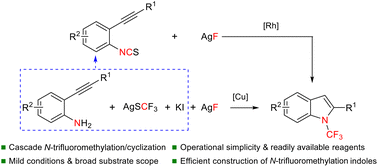
Indoles, as important N-heteroaromatic skeletons, are widely used in the fields of pharmaceuticals, agrochemicals and biological sciences, but N-trifluoromethyl indoles have been rarely explored. So far, the main methods to access N-trifluoromethyl indoles include oxidative desulfurization–fluorination of dithiocarbamates and the Fischer indole synthesis via the reaction of N-CF3 hydrazine and ketones. We report herein efficient straightforward strategies for the synthesis of N-trifluoromethyl indoles through desulfurization–fluorination/cyclization of 2-alkynylaryl isothiocyanates or 2-alkynylanilines under mild conditions. These cascade reactions possess a good substrate scope and functional group compatibility, providing a variety of N-trifluoromethyl indoles in moderate to high yields.


 Please wait while we load your content...
Please wait while we load your content...