A de novo synthesis of the bisindole alkaloid geissolosimine: collective synthesis of geissoschizoline, akuammicine, (16S)-deshydroxymethylstemmadenine and Aspidospermatan alkaloids†
Abstract
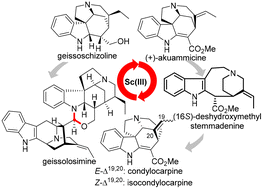
In this study, we accomplished a de novo synthesis of the bisindole alkaloid geissolosimine. Geissoschizoline, the north fragment of geissolosimine, was first prepared in 15 steps (longest linear sequence, LLS) by leveraging a catalytic asymmetric double Michael addition, which also highlights a domino oxidation/intramolecular condensation and an unprecedented SnCl4/TFA-promoted deprotection/isomerization/intramolecular cyclization cascade. The biomimetic union of geissoschizoline with vellosimine was successively realized under the influence of (±)-CSA, enabling the synthesis of geissolosimine. Additionally, this innovative strategy also provides concise access to akuammicine and the bio-inspired transformation of akuammicine via (16S)-deshydroxymethylstemmadenine to condylocarpine and iso-condylocarpine was also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...