Interception of RCONCl2: late-stage hydrolysis and esterification of primary amides†
Abstract
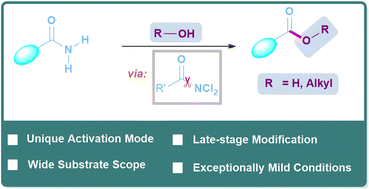
Current strategies for activating amides primarily focus on secondary or tertiary amides. However, the activation of primary amides, the simplest form of amides, remains a significant challenge. In this study, we propose a novel approach that involves generating and intercepting RCONCl2in situ to activate primary amides under mild conditions. This method allows for hydrolysis and esterification of primary amides without the use of strong Lewis acid or transition-metal catalysts typically used in amide activation. We demonstrate the synthetic potential of this approach through late-stage modifications on complex molecules, tolerance toward over 20 heterocycles, and scalability. Mechanistic experiments and DFT calculations suggest that the formation of RCONCl2 and subsequent nucleophilic attack with hydrogen bonding assistance play crucial roles in this transformation.


 Please wait while we load your content...
Please wait while we load your content...