Gold catalysed regio- and chemoselective azo coupling of 1,2- and 1,4-diazoquinones with 1H-indoles†
Abstract
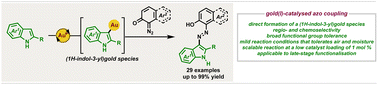
A synthetic method to prepare (E)-3-arylazoindoles efficiently that relies on the gold(I)-catalysed azo coupling of 1,2- and 1,4-diazoquinones with 1H-indoles under mild reaction conditions that did not require the exclusion of air or moisture is described. The suggested azo coupling mechanistic pathway delineates the first instance of a α-diazocarbonyl compound acting as a N-centred electrophile instead of undergoing decomposition of the dinitrogen functional group. Experimental studies based on a (1H-indol-3-yl)gold species that is proposed to be preferentially generated from the direct reaction of the group 11 metal complex and N-heterocycle over α-diazocarbonyl compound decomposition provides insight into this observed product regio- and chemoselectivity. The utility of the synthetic method was exemplified by the efficient preparation of one example at the 2 mmol scale at a catalyst loading of 1 mol %, assembly of two known (E)-diazene-based molecular photoswitches and late-stage functionalisation of two biologically relevant molecules.
- This article is part of the themed collection: 2023 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...