Halogen bonding and mechanochemistry combined: synthesis, characterization, and application of N-iodosaccharin pyridine complexes†
Abstract
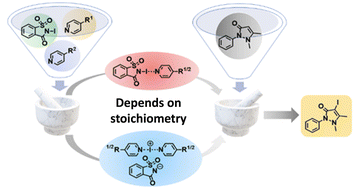
Halogen-bonded complexes are utilized across a myriad of synthetic chemistry fields, with halogen(I) complexes such as Barluenga's reagent being ubiquitous in halogenation reactions. The preparation of Barluenga's reagent requires the use of heavy metal salts and vast amounts of chlorinated solvents. In line with a more modern, environmentally conscious ethos, halogen-bonded adducts and a halogen(I) complex similar to Barluenga's reagent based on N-iodosaccharin were prepared by mechanochemical processes for the first time. The general absence of solvents or the use of vanishingly small amounts of ethyl acetate in a liquid-assisted grinding approach during mechanochemical preparations enabled the homoleptic [(DMAP)–I–(DMAP)]+ iodine(I) complex to be synthesized. The as prepared mechanochemical materials were used in the iodination of antipyrine, demonstrating their potential use as surrogates for Barluenga's reagent in both solution and solid-state syntheses.


 Please wait while we load your content...
Please wait while we load your content...