Metal-catalyzed stereoselective ring-opening polymerization of functional β-lactones: methylene-alkoxy-fluorinated polyhydroxyalkanoates unveil the role of non-covalent interactions†
Abstract
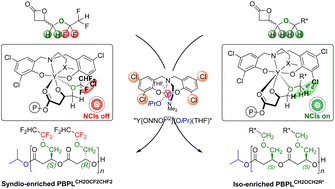
Non-covalent interactions (NCIs) can play a major role in the stereoselective ring-opening polymerization (ROP) of racemic β-lactones mediated by achiral metal catalysts, towards the formation of the corresponding polyhydroxyalkanoates (PHAs) with a specific microstructure. Our longstanding endeavor to better understand the factors governing the stereoselective ROP of 4-substituted β-propiolactone monomers rac-BPLFGs (resulting in PBPLFGs; FG = (non)functional group), from catalyst systems based on diamino- or amino-alkoxy-bis(ortho,para-substituted)phenolate rare earth complexes associated with an exogenous alcohol as an initiator, indicated that the formation of iso-enriched polyesters requires the use of ortho-halogen-substituted phenolate ligands (halogen = F, Cl, and Br) along with a methylene alkoxide pending substituent (FG = –CH2OCH2R*) on the lactone. Any other ligand/β-lactone combination resulted in either syndiotactic or atactic PBPLFGs. We report herein the controlled ROP of 4-methylene-alkoxy-fluorinated substituted β-propiolactone, rac-BPLCH2OCF2CHF2, catalyzed by diamino-bis(ortho,para-R,R-substituted)phenolate yttrium catalyst systems Y{ONNOR2}/iPrOH (with R = Me, Cl, tBu, cumyl; namely 1a–d/iPrOH, respectively). This monomer does not feature any outer methylene hydrogen in the alkoxide moiety (–CH2O![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) 2CHF2vs. –CH2O
2CHF2vs. –CH2O![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2R*), thus enabling the evaluation of the role of outer CH2O(R*)
2R*), thus enabling the evaluation of the role of outer CH2O(R*)![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2⋯R NCIs in the stereocontrol. The tBu catalyst 1c showed the highest ROP activity (TOF up to 4650 h−1) out of the four catalyst systems investigated. The fluoroalkyl PHAs were recovered with molar mass values up to Mn,NMR = 106 000 g mol−1 and narrow dispersity (ĐM = 1.02–1.24). Detailed NMR and mass spectrometry characterization studies supported the formation of α-isopropoxy,ω-hydroxy telechelic PBPLCH2OCF2CHF2 chains. Catalysts 1c and 1d flanked with bulky phenolate substituents (R = tBu, cumyl) and the chloro-substituted one 1b all returned syndio-enriched PBPLCH2OCF2CHF2s with Pr up to 0.87, as assessed by 13C NMR spectroscopy. Only the methyl substituted catalyst 1a gave atactic PHAs. Eventually, these findings support the hypothesis that both inner and outer methylene hydrogens within an alkoxymethylene exocyclic group on the rac-β-lactone monomer (
2⋯R NCIs in the stereocontrol. The tBu catalyst 1c showed the highest ROP activity (TOF up to 4650 h−1) out of the four catalyst systems investigated. The fluoroalkyl PHAs were recovered with molar mass values up to Mn,NMR = 106 000 g mol−1 and narrow dispersity (ĐM = 1.02–1.24). Detailed NMR and mass spectrometry characterization studies supported the formation of α-isopropoxy,ω-hydroxy telechelic PBPLCH2OCF2CHF2 chains. Catalysts 1c and 1d flanked with bulky phenolate substituents (R = tBu, cumyl) and the chloro-substituted one 1b all returned syndio-enriched PBPLCH2OCF2CHF2s with Pr up to 0.87, as assessed by 13C NMR spectroscopy. Only the methyl substituted catalyst 1a gave atactic PHAs. Eventually, these findings support the hypothesis that both inner and outer methylene hydrogens within an alkoxymethylene exocyclic group on the rac-β-lactone monomer (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2O
2O![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2R*) are required, along with an ortho-chloro-substituted yttrium bisphenolate catalyst (Y{ONNOCl2}), to induce NCIs (Cl⋯
2R*) are required, along with an ortho-chloro-substituted yttrium bisphenolate catalyst (Y{ONNOCl2}), to induce NCIs (Cl⋯![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2
2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) -O-
-O-![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) (R*)
(R*)![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2⋯Cl), ultimately resulting in unique isotactic synthetic PHAs.
2⋯Cl), ultimately resulting in unique isotactic synthetic PHAs.


 Please wait while we load your content...
Please wait while we load your content...