Structural flexibility of favipiravir and its structural analogues in solutions: experimental and computational insight†
Abstract
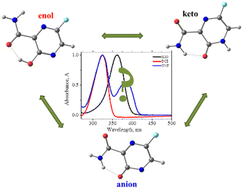
Combined UV-vis and quantum chemical studies of the structural flexibility and tautomerism of 6-R-3-hydroxy-2-pyrazine carboxamides in solutions revealed that their keto–enol transformations are accompanied by the deprotonation of enol tautomers and the formation of the corresponding anionic species. Both the solvent and the 6-R substituent strongly influence the relative abundance of the above forms in solutions. Anions are not formed in 1,2-dichloroethane (DCE), but the probability of deprotonation in neutral water and N,N-dimethylformamide (DMF) increases in the order R = H < F < NO2. Only enol tautomers of all solutes are found in DCE. DMF stabilizes keto forms only moderately and assists much strongly in the deprotonation of all three compounds. Water tends to stabilize both keto tautomers and deprotonated anions: the keto form dominates in the case of R = H (antiviral drug T-1105), the anions are found exclusively for R = NO2, and the aqueous solution of another antiviral drug, favipiravir (R = F), contains both the keto tautomer and the anionic form. The results of quantum chemical free energy calculations are in agreement with the experimental observations.


 Please wait while we load your content...
Please wait while we load your content...