BODIPY(aryl)iodonium salts in the efficient synthesis of diversely functionalized BODIPYs and selective detection of serum albumin†
Abstract
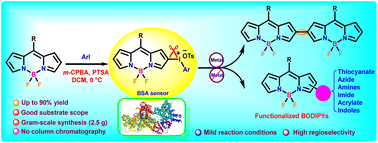
BODIPY(aryl)iodonium salts were readily accessible from the high-yielding reaction of BODIPY with iodoarenes or hydroxyl(tosyloxy)iodoarenes in the presence of m-CPBA. The prepared BODIPY(aryl)iodonium salts bearing substituents of varied electronic nature were utilized for the direct syntheses of thiocyanate, azide, amine and acrylate functionalized BODIPYs and β,β′-bis-BODIPYs. The regioselective syntheses of α-piperidinyl and β-piperidinyl substituted BODIPYs were achieved through the reaction of BODIPY(aryl)iodonium salts with piperidine in the absence and presence of copper(I). Expeditious and high yielding (79–82%) synthesis of β,β′-bis-BODIPYs was also developed through the palladium-catalyzed reductive coupling of the easily accessible BODIPY(aryl)iodonium salts. Some of the indole-appended BODIPYs and bis-BODIPYs displayed strong absorption in the visible region (∼610 nm). The BODIPY(aryl)iodonium salts also showed significant binding with serum albumin and were observed to be selective serum protein sensors with estimated limits of detection as low as 7 μg mL−1 in some cases.


 Please wait while we load your content...
Please wait while we load your content...