Rhodium-catalysed additive-free alkoxycarbonylation of indoles: 2,4,6-trimethylbenzoic acid-based carbonate anhydrides as a versatile alkoxycarboxyl source†
Abstract
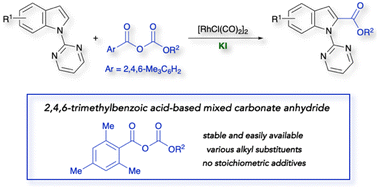
We report a CO-free approach to indole-2-carboxylic esters: rhodium-catalysed C(2)-alkoxycarbonylation of indoles with 2,4,6-trimethylbenzoic acid-based carbonate anhydrides. Selective C–O bond cleavage of the anhydrides facilitates the introduction of various alkoxycarbonyl groups. Control experiments suggest that merging a rhodium catalyst and KI promotes the in situ formation of the RhI species.


 Please wait while we load your content...
Please wait while we load your content...