Identification and quantification of N6-methyladenosine by chemical derivatization coupled with 19F NMR spectroscopy†
Abstract
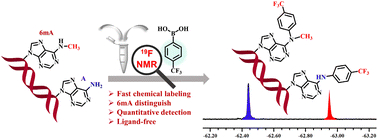
N 6-Methyladenosine (6mA) is a well-known prokaryotic DNA modification that has been shown to play epigenetic roles in eukaryotic DNA. Accurate detection and quantification of 6mA are prerequisites for molecular understanding of the impact of 6mA modification on DNA. However, the existing methods have several problems, such as high false-positive rate, time-consuming and complex operating procedures. Chemical sensors for the selective detection of 6mA modification are rarely reported in the literature. Fluorinated phenylboronic acid combined with 19F NMR analysis is an effective method for determining DNA or RNA modification. In this study, we presented a simple and fast chemical method for labelling the 6th imino group of 6mA using a boric-acid-derived probe. Besides, the trifluoromethyl group of trifluoromethyl phenylboronic acid (2a) could detect 6mA modification through 19F NMR. Combined with this sensor system, 6mA modification could be detected well and quickly in 6 types of deoxynucleoside mixtures and DNA samples. Taken together, the method developed in the current study has potential for specific detection of 6mA in biological samples.


 Please wait while we load your content...
Please wait while we load your content...