Electrochemical selenofunctionalization of unactivated alkenes: access to β-hydroxy-selenides†
Abstract
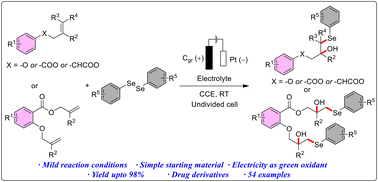
This work demonstrates the electrochemical construction of 2-methyl-1-aryloxy-3-(arylselanyl)propan-2-ol/2-hydroxy-2-methyl-3-(arylselanyl)propyl 2-(2-hydroxy-2-methyl-3-(arylselanyl)propoxy)benzoate starting from aryl allyl ethers/allyl benzoates and diaryl diselenides under additive-free electrochemical conditions. This environmentally friendly method was achieved through constant current electrolysis in an undivided cell setup under acid, oxidant, or catalyst-free conditions. Additionally, this technique enabled the synthesis of a variety of β-hydroxy selenides including late-stage functionalization of drug derivatives in good to exceptional yields across various substrates under mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...