Visible-light-induced C–S bond formation in the synthesis of 2,4-disubstituted thiazoles through cascade difunctionalization of acetophenone: a greener approach†
Abstract
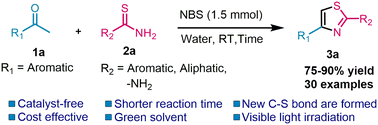
A groundbreaking approach has been developed for synthesizing 2,4-disubstituted thiazoles using an eco-friendly and metal-free approach. This novel method utilizes methyl aryl ketones, N-bromo-succinimide (NBS), and thioamides in water as a green reaction medium under visible light irradiation. Using NBS as a bromine source, the reaction takes place through an in situ α-bromination method. This approach does not require any catalyst, which makes it exceptionally beneficial for the environment. The advantages of this efficient approach are manifold and include the use of greener conditions, absence of metals, easy isolation of products, cost-effectiveness, non-toxicity, and reliance on renewable energy sources like visible light. Moreover, this technique offers higher product purity and excellent yield, enhancing itsappeal.


 Please wait while we load your content...
Please wait while we load your content...