Direct synthesis of conjugated tetraenes from 1,3-enynes with 1,3-dienes†
Abstract
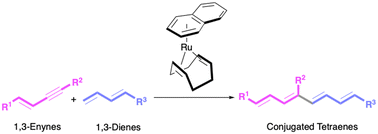
New direct access to conjugated tetraenes has been achieved. A Ru(0)-catalysed reaction of 1,3-enynes with 1,3-dienes gives 1,3,5,7-octatetraene derivatives by formal regioselective insertion of the alkynyl group of 1,3-enynes into the terminal C–H bond in 1,3-dienes. With a silyl substituent on the alkynyl side in 1,3-enynes, the reaction regioselectively proceeds to give the linear cross-dimerisation product having the silyl group at the internal position. Stoichiometric and DFT calculations support the oxidative coupling mechanism for the linear cross-dimerisation. Methyl (2E,4E,6E,8E)-10-hydroxy-2,4,6,8-decatetraenoate, a versatile polyene intermediate, is accessed by this method as a formal synthesis of biologically active compounds.


 Please wait while we load your content...
Please wait while we load your content...