Selective electrochemical acceptorless dehydrogenation reactions of tetrahydroisoquinoline derivatives†
Abstract
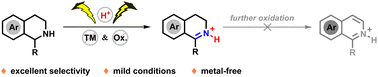
Selective dehydrogenation reactions of tetrahydroisoquinoline derivatives through electrochemical oxidation are disclosed. In the presence of nitric acid, the selective partial dehydrogenation of tetrahydroisoquinolines to form 3,4-dihydroisoquinolines was achieved via anodic oxidation. The results of CV (Cyclic Voltammograms) experiments and DFT calculations showed the 3,4-dihydroisoquinolines protonated by an external Brønsted acid to be less prone than their unprotonated counterparts to oxidation under electrochemical conditions, thus avoiding their further dehydrogenation. Moreover, a TEMPO-mediated electrochemical oxidation enabled a complete dehydrogenation to yield fully aromatized isoquinolines. Thus, tunable processes involving electrochemical dehydrogenation of tetrahydroisoquinolines could be used to selectively produce various 3,4-dihydroisoquinolines and isoquinoline derivatives.


 Please wait while we load your content...
Please wait while we load your content...