Organocatalytic enantioselective Mannich and retro-Mannich reactions and combinations of these reactions to afford tetrasubstituted α-amino acid derivatives†
Abstract
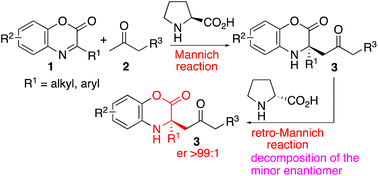
Organocatalytic asymmetric Mannich reactions and kinetic resolutions of the products via retro-Mannich reactions that afford enantiomerically enriched tetrasubstituted α-amino acid derivatives (α,α-disubstituted-α-amino acid derivatives) were developed. Furthermore, the combination of the Mannich reaction and the retro-Mannich reaction allowed access to products with almost perfect enantiopurities.


 Please wait while we load your content...
Please wait while we load your content...