Substituent-controlled regioselective arylation of carbazoles using dual catalysis†
Abstract
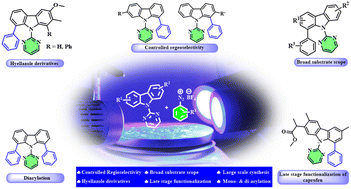
Regioselective arylation of carbazoles is reported using dual palladium–photoredox catalysis. Controlled monoarylation and diarylation of symmetrical and unsymmetrical carbazoles were achieved under mild reaction conditions with a broad substrate scope and functional group tolerance. Steric and electronic control the regioselectivity of the arylation of unsymmetrical carbazoles. Late-stage functionalization of a caprofen drug derivative and large-scale synthesis of mono- and di-arylated carbazoles were demonstrated to showcase the synthetic versatility of the method. Finally, we also showcased the synthesis of hyellazole analogues (a marine alkaloid) in a short route using our strategy.


 Please wait while we load your content...
Please wait while we load your content...