Base controlled rongalite-mediated reductive aldol/cyclization and dimerization of isatylidene malononitriles/cyanoacetates†
Abstract
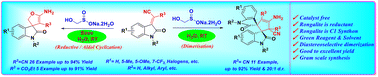
In this study, we developed a novel methodology involving a base-controlled, rongalite-mediated reductive/aldol reaction, followed by cyclization of isatylidene malononitriles/cyanoacetates, resulting in the synthesis of spiro[2,3-dihydrofuran-3,3′-oxindole]. Additionally, we have disclosed a rongalite-mediated dimerization process for isatylidene malononitriles, yielding dispiro[cyclopent-3′-ene]bisoxindole. The utilization of rongalite in this reaction serves a dual purpose, acting both as a reducing agent and a C1 synthon. The developed approach has several advantages like a simple reaction setup, a wide substrate scope, requiring less time, using water as a green solvent, no metal or catalyst is required and products can be easily isolated via filtration with excellent yields under mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...