Nickel(ii)/Lewis acid catalysed olefin hydroamination and hydroarylation under mild conditions†
Abstract
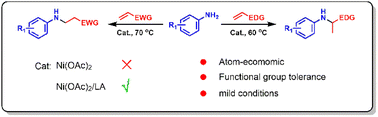
Aniline derivatives are important nitrogen-containing compounds with wide applications in chemicals, pharmaceuticals and agrochemicals. In the work described herein, nickel(II)/Lewis acid (LA) catalysed olefin hydroamination with anilines was explored for use in aniline derivative syntheses. The Ni(II)/LA catalysis proceeded smoothly under mild conditions, whereas using Ni(OAc)2 alone, the catalyst was inactive. Remarkably, the Markovnikov addition type products were obtained when substituted styrenes were used as the olefin source, while the anti-Markovnikov addition type products were obtained when the electron-deficient olefins such as acrylonitrile and acrylates were used. The mechanistic studies revealed that hydroamination of the styrene derivates proceeded via the amino-Ni(II)/LA attacking the carbocation intermediate which was generated by the protonation of the olefin, whereas for acrylonitrile and acrylates, it proceeded by a direct amino-Ni(II)/LA attack on the olefin by nucleophilic addition. In addition, the hydroarylation product was generated by the Hofmann–Martius rearrangement of the hydroamination product.


 Please wait while we load your content...
Please wait while we load your content...