Concise approach to γ-(het)aryl- and γ-alkenyl-γ-aminobutyric acids. Synthesis of vigabatrin†
Abstract
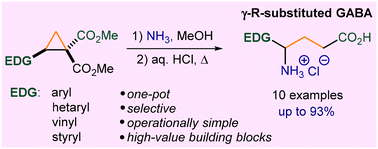
γ-Aminobutyric acid (GABA) and GABA derivatives have attracted increased attention over the years in the fields of medicinal chemistry and chemical biology due to their interesting biological properties and synthetic relevance. Here, we report a short synthetic route to γ-(het)aryl- and γ-alkenyl-γ-aminobutyric acids, including the antiepileptic drug vigabatrin, from readily available donor–acceptor cyclopropanes and ammonia or methylamine. This protocol includes a facile synthesis of 2-oxopyrrolidine-3-carboxamides and their acid hydrolysis to γ-aryl- or γ-alkenyl-substituted GABAs, which can serve as perspective building blocks for the synthesis of various GABA-based N-heterocycles and bioactive compounds.


 Please wait while we load your content...
Please wait while we load your content...