Recent advances in ring-opening of cyclobutanone oximes for capturing SO2, CO or O2via a radical process
Abstract
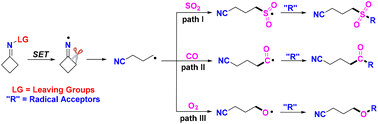
Cyclobutanone oximes and their derivatives are pivotal core structural motifs in organic chemistry. Iminyl-radical-triggered C–C bond cleavage of cyclobutanone oximes delivers an efficient strategy to produce stable distal cyano-substituted alkyl radicals, which can capture SO2, CO or O2 to form cyanoalkylsulfonyl radicals, cyanoalkylcarbonyl radicals or cyanoalkoxyl radicals under mild conditions. In the past several years, cyanoalkylsulfonylation/cyanoalkylcarbonyaltion/cyanoalkoxylation has attracted a lot of interest. In this updated report, the strategies for trapping SO2, CO or O2via iminyl-radical-triggered ring-opening of cyclobutanone oximes are summarized.


 Please wait while we load your content...
Please wait while we load your content...