Structural and optical control through anion and cation exchange processes for Sn-halide perovskite nanostructures†
Abstract
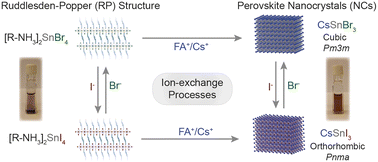
Metal halide perovskite nanostructures, characterized by their ionic nature, present a compelling avenue for the tunability of dimensions and band gaps through facile compositional transformations involving both cationic and anionic exchange reactions. While post-synthetic ion-exchange processes have been extensively explored in Pb-halide perovskite nanocrystals, the inherent instability of Sn2+ has limited the exploration of such processes in Sn-halide perovskite nanostructures. In this study, we present a straightforward cation exchange process wherein 2D [R–NH3]2SnX4 Ruddlesden–Popper (RP) nanostructures with n = 1 transition to 3D ASnX3 nanocrystals at room temperature with the addition of A-cation oleate. In addition, anion exchange processes have been demonstrated for both 2D [R–NH3]2SnX4 RP nanostructures and 3D nanocrystals, showcasing transitions between iodide and bromide counterparts. Furthermore, we have fabricated a thin film of 2D [R–NH3]2SnX4 RP nanostructures for cation exchange, wherein A-cation diffusion through a liquid–solid interface facilitates the transformation into a 3D ASnX3 crystal. This investigation underscores the versatility of ion exchange processes in engineering the composition of Sn-halide perovskite nanostructures and, consequently, modulating their optical properties.
- This article is part of the themed collections: Nanoscale 2024 Emerging Investigators and Nanoscale Most Popular 2024 Articles


 Please wait while we load your content...
Please wait while we load your content...