Design, synthesis and evaluation of structurally diverse polycyclic harmaline scaffolds as anticancer agents†
Abstract
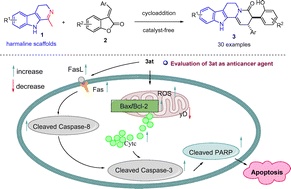
Here, we demonstrate that the first example of harmaline scaffolds serving as acceptor/acceptor-based N–C–C synthons triggered the ring opening and skeletal reconstruction of 3-vinyl benzofuranones for the construction of a library of skeletally diverse polycyclic harmaline scaffolds. These products were smoothly afforded in up to 77% yield, >20 : 1 dr under catalyst-free conditions, and expanded the chemical space of biologically significant harmaline derivative species. All synthetic compounds showed cytotoxic activity against tumor cells, especially compound 3at, which exhibited selective cytotoxicity against A549 cells and lower cytotoxicity against normal cells MRC-5 and HK-2. In further mechanism research, 3at inhibited A549 cell proliferation through the G1 phase arrest, induced A549 cell apoptosis through internal and external apoptosis pathways, and suppressed the migration and invasion abilities of A549 cells. These data indicated that compound 3at is the most effective compound in the series and can serve as a promising precursor for drug development.


 Please wait while we load your content...
Please wait while we load your content...