Catalytic activity of cyclobutadiene rhodium complexes in hydrosilylation and other transformations of alkynes†
Abstract
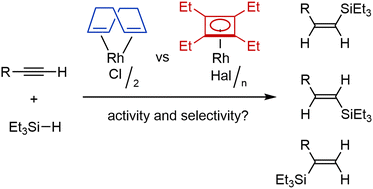
The catalytic activity of cyclobutadiene rhodium complexes was investigated in various transformations of alkynes. Complexes [Rh(C4Et4)Hal]n (at 1 mol% loading) were found to promote hydrosilylation of 1-octyne selectively giving 1-Z-, 1-E-, or 2-Et3Si-1-octene depending on the nature of the halide ligand and the presence of additional ligand PPh3. Complex [Rh(C4Et4)(p-xylene)]+ (1 mol%) also efficiently catalyzed the polymerization of phenylacetylene. At the same time, we found that various cyclobutadiene rhodium complexes do not catalyze the Diels–Alder reaction, alkyne hydration, and 1,4-addition of boronic acids to cyclohexanone. New complexes [Rh(C4Et4)(PPh3)2]BF4 and [Rh(C4Et4)(C10H8)]PF6 were also synthesized and their structures were established by X-ray diffraction.


 Please wait while we load your content...
Please wait while we load your content...