Conjugates of titanocene with non-steroidal anti-inflammatory drugs: synthesis, unusual NMR characteristics, stability and cytotoxicity†
Abstract
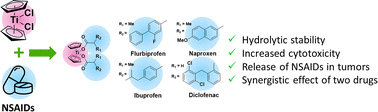
Titanocene conjugates with four different non-steroidal anti-inflammatory drugs (flurbiprofen, naproxen, ibuprofen, and diclofenac; NSAIDs) were obtained for the first time. The spectra of the obtained compounds were recorded, and an unexpectedly strong effect of chiral ligands based on NSAIDs on the chemical shifts of protons of cyclopentadienyl rings of titanocene in 1H NMR spectra was discovered and explained. For all the obtained conjugates, the redox behavior in DMF solution was investigated; the reduction potentials of all titanocene–NSAID compounds were in the range of −0.80 to −0.98 V, which were suitable for their intracellular reduction. The stability of all the conjugates obtained in aqueous media turned out to be many times higher than the stability of titanocene dichloride (the half-life of titanocene–NSAID conjugates was 50–180 minutes, and for one of the conjugates it exceeded 400 minutes). The most cytotoxic of the obtained compounds, the titanocene conjugate with ibuprofen, showed IC50 against the A2780 cell line 35% lower than the original titanocene dichloride and SI = 1.54 in relation to non-tumor human fibroblast cells.


 Please wait while we load your content...
Please wait while we load your content...