Mg-doped cathodic properties and solid-state ionic conduction in P2-type layered material for Na-ion batteries and supercapacitors†
Abstract
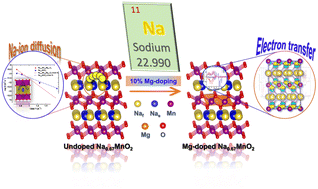
Sodium-ion batteries and supercapacitors look promising candidates as an alternative solution for electrochemical energy storage due to their decent energy density, low cost, good reversibility, and high abundance on the Earth's surface. Sodium-ion batteries exhibit analogous performance to lithium-ion batteries, with the requirements of essential electrolytes and electrode materials. Understanding ionic diffusion in cathode materials is crucial. Here, we study P2-layered Na0.67MnO2 and with 10% Mg-doping, i.e., Na0.67Mn0.9Mg0.1O2, as cathode materials using first-principles calculations and classical molecular dynamics (MD) simulations. We report their ionic diffusion and conduction mechanism using MD simulation methods. We report an enhancement in the transport properties of cathode materials by partial substitution of Mg in Mn octahedral sites, which accelerates the diffusion of Na ions and high-conductivity Na+ ions compared to Na0.67MnO2. Such behavior in an Na-ion battery cathode material focuses on high-availability metals Mn, Mg, and Na. We summarise the results of various computational methods in finding the optimized structure, electronic calculations, cathodic properties, and diffusion properties. Our study will help us understand the Na chemistry involved in developing cathode materials for large-scale applications.


 Please wait while we load your content...
Please wait while we load your content...