Potassium energy storage behavior of nickel–zinc co-doped Prussian blue analogs formed by a chelating-agent-assisted route and its application in a K+-proton hybrid-ion aqueous alkaline battery†
Abstract
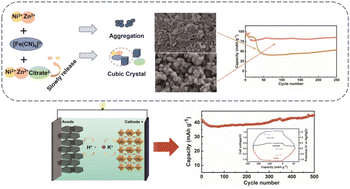
A series of nickel–zinc hexacyanoferrate (NixZnyHCF) was synthesized through a conventional co-precipitation method, and their potassium-ion storage performances were tested in a 30% KOH solution. Among these samples, Ni3Zn1HCF obtained the highest capacity (96.7 mA h g−1). After introducing the chelating agent in the synthetic procedure, the obtained Ni3Zn1HCF-10 sample displayed high crystallinity with reduced crystal water and improved cyclic stability (99.0% capacity retention after 350 cycles) over the sample prepared without involving the chelating agent (56.2% capacity retention after 250 cycles). Finally, a K+/H+ hybrid-ion aqueous alkaline battery was built with a Ni3Zn1HCF-10 cathode and an active carbon anode. The constructed full cell delivered a capacity of 44.8 mA h g−1 at a 1.6 V operational voltage. Further, it had excellent rate capability and low self-discharge along with good stability (99.6% capacity retention after 500 cycles). This study provides a great strategy for fabricating efficient rechargeable aqueous alkaline batteries.


 Please wait while we load your content...
Please wait while we load your content...