Cu-catalyzed cycloaddition of aryl azides to 1-iodobuta-1,3-diynes: an experimental and quantum chemical study of unusual regiochemistry†‡
Abstract
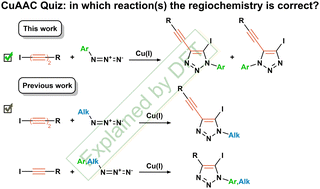
Cu-catalyzed azide–alkyne cycloaddition (CuAAC) in the case of 1-iodoalkynes is known as a synthetic tool towards 5-iodo-1,2,3-triazole derivatives. We found that CuAAC of 1-iodobuta-1,3-diynes and aryl azides under CuI(PPh3)3 catalysis unexpectedly leads to the formation of both 4-iodo- and 5-iodo-1,2,3-triazoles. Aryl azides bearing acceptor groups and iodoaryldiacetylenes having donor groups shift the isomer ratio in favor of nontrivial 4-iodotriazoles. The reason for the change in the regioselectivity was explained using DFT calculations, which revealed the binuclear nature of the CuAAC transition states (TSs) for iodoalkynes and azides cycloaddition. The regiochemistry of cycloaddition is determined by the type of azide N atom coordinated to the Cu atom and by a spatial arrangement of the binuclear Cu catalyst and alkyne in the TS. In particular in the case of 1-iodobuta-1,3-diynes both regioisomeric TSs have a linear orientation of the alkyne moiety and the I–Cu–P fragment of the binuclear catalyst that makes both N1–Cu (for 5-I-TS) and N3–Cu (for 4-I-TS) coordination possible. The influence of various electronic and stereoelectronic effects established by NBO analysis, as well as NCI interactions, on the stabilization of isomeric TSs in the reactions of aryl/alkyl azides with iodomono- and diacetylenes is discussed. 4-Iodotriazoles are more thermodynamically stable than 5-iodotriazoles, while only the latter form I–N halogen bonds in the solid state.


 Please wait while we load your content...
Please wait while we load your content...