Harnessing prion-inspired amyloid self-assembly for sustainable and biocompatible proton conductivity†
Abstract
Protein-based materials have emerged as promising candidates for proton-conducting biomaterials. Therefore, drawing inspiration from the amino acid composition of prion-like domains, we designed short self-assembling peptides incorporating the (X-Tyr) motif, with X representing Asn, Gly and Ser, which form fibrillar structures capable of conducting protons. In this study, we conducted an analysis of the conductivity capacity of these fibers, with a focus on temperature and frequency dependence of conductivity. The loss tangent curves data and the electrode polarization model with the Debye approximation were employed to calculate transport properties, including conductivity, diffusivity, and density of charge carriers. Results revealed the prion-like fibers can transport protons more efficiently than biomaterials and other synthetic proton conducting materials, and that a significant increase in conductivity is observed with fibrillar orientations. The temperature dependence of conductivity of the peptides, measured in wet conditions, showed conductivities following the trend σ(NY7) < σ(GY7) < σ(SY7), in all the range of temperatures studied. The Arrhenius behavior, and the activation energy associated with conductivity followed the trend: Eact (SY7) = 8.2 ± 0.6 kJ mol−1 < Eact (GY7) < 13 ± 5 kJ mol−1 < Eact (NY7) = 31 ± 7 kJ mol−1, in different range of temperatures depending of the peptide. Furthermore, the diffusion coefficient correlated with increasing temperature in GY7 and SY7 fibers for temperatures compress between 20 °C and 80 °C, while NY7 only below 60 °C. However, it is noteworthy that the diffusivity observed in the SY7 peptide is lower, compared to GY7 and NY7 presumably due to its enlarged length. This observation can be attributed to two factors: firstly, the higher conductivity values observed in SY7 compared to GY7 and NY7, and secondly, to the value of relation 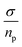 observed of cations present in the peptide SY7 compared with GY7 and NY7, which in turn is dependent on temperature. In light of these findings, we envision our prion-inspired nanofibers as highly efficient proton-conducting natural biopolymers that are both biocompatible and biodegradable. These properties provide the opportunity for the development of next-generation bioelectrical interfaces and protonic devices.
observed of cations present in the peptide SY7 compared with GY7 and NY7, which in turn is dependent on temperature. In light of these findings, we envision our prion-inspired nanofibers as highly efficient proton-conducting natural biopolymers that are both biocompatible and biodegradable. These properties provide the opportunity for the development of next-generation bioelectrical interfaces and protonic devices.



 Please wait while we load your content...
Please wait while we load your content...