Protecting-group-free mechanosynthesis of amides from hydroxycarboxylic acids: application to the synthesis of imatinib†
Abstract
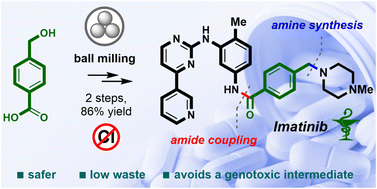
Despite considerable advancements in mechanochemical amide couplings, there is a paucity of studies addressing chemoselective issues in these transformations, such as the tolerance of unmasked hydroxyl groups. In view of the high practical significance of amide coupling reactions in the synthesis of active pharmaceutical ingredients (APIs), we aimed to investigate the tolerance of unprotected hydroxyls in carboxylic acids towards various reported mechanochemical amide coupling conditions. 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl) in combination with ethyl acetate as a liquid-assisted grinding (LAG) additive was revealed as the most selective amide coupling system that delivers 76–94% yields of amides from a range of hydroxycarboxylic acids, including N-Boc-protected amino acids serine and tyrosine. The EDC-mediated amide coupling protocol was employed in the synthesis of imatinib, an anticancer drug included in the World Health Organization's List of Essential Medicines. The target API was synthesized in an overall 86% yield and 99% HPLC purity through a two-step mechanochemical C–N bond assembling reaction sequence starting from 4-(hydroxymethyl)benzoic acid.


 Please wait while we load your content...
Please wait while we load your content...