Mechanically induced self-propagating reactions (MSRs) to instantly prepare binary metal chalcogenides: assessing the influence of particle size, bulk modulus, reagents melting temperature difference and thermodynamic constants on the ignition time†
Abstract
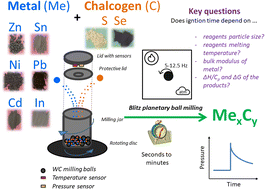
Mechanically induced self-propagating reactions (MSRs) offer the possibility to obtain desired products in an ultrafast and thus cost- and energy-efficient manner. In this work, this is demonstrated for ten binary metal chalcogenides (CdS, CdSe, In2S3, NiS, NiSe, PbS, PbSe SnSe, ZnS and ZnSe) by processing mixtures of metals and chalcogens in a planetary ball mill for less than 10 minutes. The MSR process for Ni-based systems is reported for the first time. The studied metals reacted much faster with selenium than with sulfur (with the exception of Ni). The successful MSR occurrence was evidenced by an abrupt increase of gas pressure in the milling jar monitored in situ and subsequently ex situ by X-ray diffraction. The crystallite size of the as-received products was usually in the range 40–260 nm. All the reactions were performed in an air atmosphere, and thus the presence of an inert gas was not necessary. An effort was made to correlate the observed ignition times with the particle size of the precursors, their melting temperature, bulk modulus and thermodynamic parameters (ΔH/Cp and ΔG). While the thermodynamics does not seem to play an important role here, the particle size and bulk modulus of the reacting metal most probably influence the ignition time of MSRs. Namely, higher ductility (low bulk modulus) and finer particles seem to shorten the activation period before the MSR ignition. This study forms a cornerstone for further research in MSRs of metal chalcogenides because it universally assesses the influence of more parameters on the MSR course under fixed milling conditions for different systems. The proposed synthetic pathway also represents an improvement by reducing both time and energy by showing the possibility to reach some of the desired products within a minute-range, being much faster than a classical gradual reaction and further underlines the environmentally benign character of mechanochemistry.
- This article is part of the themed collection: RSC Mechanochemistry 2024 Hot Articles


 Please wait while we load your content...
Please wait while we load your content...