Recognition of arylmethylidene derivatives of imidazothiazolotriazinones as novel tubulin polymerization inhibitors†
Abstract
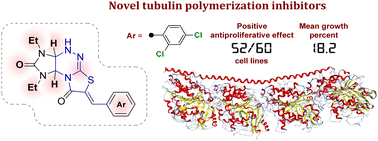
Two series of arylmethylidene derivatives of imidazothiazolotriazinone differing in the structure of the imidazothiazolotriazine fragment were synthesized and their antiproliferative activity and effect on tubulin polymerization were evaluated. Some of the synthesized derivatives showed a significant antiproliferative effect, among which (Z)-7-(2,4-dichlorobenzylidene)-1,3-diethyl-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-2,8(3H,7H)-dione 2n exhibited the highest antiproliferative activity. The GI50 values of the compound against 56 of the 58 cell lines were 19.4–87.8 nM; against the remaining 2 cell lines, they were 0.544–1.29 μM. Moreover, further mechanism analysis demonstrated that 2n caused G2/M arrest, induced cell apoptosis in K562 cells and blocked tubulin polymerization in the same way as colchicine.


 Please wait while we load your content...
Please wait while we load your content...