Small molecule WDR5 inhibitors down-regulate lncRNA expression†
Abstract
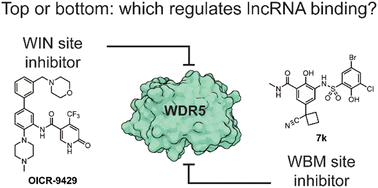
WD repeat domain 5 (WDR5) plays an important role as a scaffold protein in both protein–protein and RNA–protein complexes involved in epigenetic gene regulation. In particular, some of these lncRNAs were reported to regulate the expression of genes in cis as well as themselves through binding WDR5. In this report, we investigate the two known binding sites of WDR5 in relation to lncRNA binding and expression. The WBM binding site mediates both protein–protein and lncRNA–protein interactions while the WIN site, which is on the opposite side of the protein, is only known to mediate protein–protein interactions. To dissect the function of different binding sites on WDR5, we characterized them with selective peptide ligands using fluorescence polarization and used these to demonstrate the selectivity of small molecule inhibitors of these two major binding sites. RNA immunoprecipitation experiments were performed to show that lncRNA–WDR5 complex formation could be interrupted using a WBM site inhibitor. Finally, we demonstrated that WDR5 regulated lncRNAs are down regulated with different sensitivity toward the corresponding inhibitors, demonstrating the potential of targeting lncRNA–protein interactions to reduce oncogenic lncRNA expression.
- This article is part of the themed collection: Targeting RNA


 Please wait while we load your content...
Please wait while we load your content...