In vivo stability of 211At-radiopharmaceuticals: on the impact of halogen bond formation†
Abstract
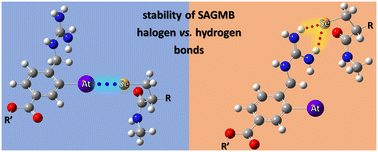
211At, when coupled to a targeting agent, is one of the most promising radionuclides for therapeutic applications. The main labelling approach consists in the formation of astatoaryl compounds, which often show a lack of in vivo stability. The hypothesis that halogen bond (XB) interactions with protein functional groups initiate a deastatination mechanism is investigated through radiochemical experiments and DFT modelling. Several descriptors agree on the known mechanism of iodoaryl substrates dehalogenation by iodothyronine deiodinases, supporting the higher in vivo dehalogenation of N-succinimidyl 3-[211At]astatobenzoate (SAB) conjugates in comparison with their iodinated counterparts. The guanidinium group in 3-[211At]astato-4-guanidinomethylbenzoate (SAGMB) prevents the formation of At-mediated XBs with the selenocysteine active site in iodothyronine deiodinases. The initial step of At-aryl bond dissociation is inhibited, elucidating the better in vivo stability of SAGMB conjugates compared with those of SAB. The impact of astatine's ability to form XB interactions on radiopharmaceutical degradation may not be limited to the case of aryl radiolabeling.


 Please wait while we load your content...
Please wait while we load your content...