Design, synthesis, and antiproliferative evaluation of novel dehydroabietic acid-1,2,3-triazole-oxazolidinone hybrids†
Abstract
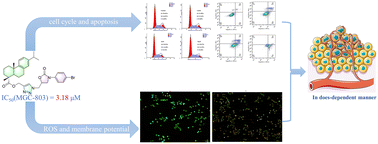
A series of novel dehydroabietic acid derivatives containing both 1,2,3-triazole and oxazolidinone 4a–4t have been synthesized and their antiproliferative activity in vitro against HeLa, HepG2, MGC-803 and T-24 cell lines evaluated. Most of them displayed cell proliferation inhibition on four tested human malignant tumour cell lines to some degree. Among them, compound 4p exhibited promising cytotoxicity with IC50 values ranging from 3.18 to 25.31 μM and weak cytotoxicity toward normal cells. The mechanism of action of 4p was then studied using flow cytometry, Hoechst 33258 staining, ROS generation assay, and JC-1 mitochondrial membrane potential staining, which illustrated that compound 4p induced apoptosis, arrested mitotic process at the G1 phase of the cell cycle, reduced the mitochondrial membrane potential, and increased intracellular ROS levels. In summary, the introduction of an oxazolidinone group via a “1,2,3-triazole” linker significantly improved the antitumor activity of dehydroabietic acid, and deserves to be further investigated.


 Please wait while we load your content...
Please wait while we load your content...