Zinc oxide–copper model nanocatalysts for CO2 hydrogenation: morphology and interface effects†
Abstract
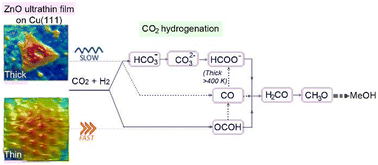
Understanding the mechanism of carbon dioxide (CO2) hydrogenation reaction (HR) to methanol is a major goal as it is an attractive approach to mitigate CO2 emissions by converting them into high-value-added chemicals. Here, we conducted a comparative study on two different ZnO thicknesses grown on Cu(111) as model catalysts. Based on X-ray Photoelectron Spectroscopy (XPS) investigations, we show that the CO2 HR proceeds via two primary paths depending on ZnO thickness: (i) it takes place through a slow path involving bicarbonates, carbonates, and formates, on a thick ZnO film (6.8 monolayers (ML)); (ii) a different and a rapid path was seen for a thin ZnO film (0.9 ML) where the carboxyls were formed readily at room temperature without the formate intermediate. The key effect in the thin ZnO film (ii) (0.9 ML ZnO coverage) involves the Zn–Cu interface which provides activated H atoms. However, both paths exhibit common intermediates as they merge into H2CO which is hydrogenated to CH3O. Additionally, better thermal stability was evidenced for the ZnO thin film (0.9 ML) owing to the presence of a Zn–Cu interface metallic alloy. We demonstrate from DFT computations that a Zn–Cu interface alloy is energetically favorable even in the presence of adsorbed oxygen atoms on Cu(111). The ZnO dewetting phenomenon observed above 550 K was mediated by the desorption of OH species, while CO2 adsorption was found to stabilize ZnO film even at high temperatures (above 550 K). From the morphology point of view, the ZnO films exhibit two distinct structures as a function of the thickness. At low coverage (0.9 ML), ZnO grows into well-ordered Moiré-like patterns with a periodicity of ∼10 Å corresponding to a ZnO-(3 × 3)/Cu(111)-(4 × 4) structure. At high coverage (6.8 ML), the transition towards the ZnO wurtzite structure occurs with the formation of equilateral triangular features on the surface. This study provides insights into the role of the morphology, especially metal–oxide thickness, and the interface in the CO2 HR mechanism.


 Please wait while we load your content...
Please wait while we load your content...