Enhancement of replacement lithography by combination of photocleavable groups with ultrashort thiolates†‡
Abstract
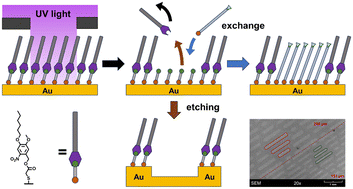
The radiation-induced replacement lithography of self-assembled monolayers (SAMs) is one of the most flexible patterning techniques in nanotechnology as it not only permits a localized substitution, but also a variation in chemistry when applied in a sequential manner. While it typically proceeds by weakening of the molecule–substrate interactions, we present here an approach in which the molecules within the SAM are cleaved, leaving behind a SAM consisting of ultrashort molecules (thioglycolic acid), which are labile enough to be efficiently replaced with a different kind of molecule. The key of this process was the introduction of a photocleavable ortho-nitrobenzyl (ONB) group carrying hexyl groups, which result in primary SAMs stable enough to withstand the carefully chosen replacement conditions. The primary SAMs were characterized by ellipsometry, infrared reflection spectroscopy (IRRAS) and contact angle goniometry, showing a somewhat surprising conformation of the SAM constituents in which the molecular dipole moment is arranged parallel to the surface. This causes the system carrying only one hexyl group to be more stable than the one with two hexyl groups. Upon irradiation with light of 365 nm, the former molecule becomes easily exchanged by (deuterated) dodecanethiol, as could be quantified by IRRAS, with an exchange yield of >80% at an area dose of 48 J cm−2. As a proof of principle, irradiation was performed with differently patterned masks, demonstrating the viability of the method for lithography.
- This article is part of the themed collections: Celebrating George Whitesides’ 85th birthday and Editor’s Choice – Jianbin Huang


 Please wait while we load your content...
Please wait while we load your content...