Visible-light-driven three-component annulation for the synthesis of highly functionalized 2-iminothiazolidin-4-ones without photocatalysts†
Abstract
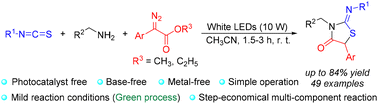
We have developed a photocatalyst/base/metal-free, visible-light-driven three-component annulation protocol to access fully substituted 2-iminothiazolidin-4-one derivatives from isothiocyanates, amines, and aryl diazoacetates using a low-energy white light source at room temperature in open air. The [2 + 2 + 1] reaction undergoes successive C–N/C–S bond formation in one pot. This transformation proceeds under extremely mild conditions with a good yield and a good functional group tolerance. Mechanistic studies showed that the reaction involves the formation of free carbene intermediates from aryl diazoacetates under visible light irradiation.


 Please wait while we load your content...
Please wait while we load your content...