Missing-linker defects in a covalent organic framework photocatalyst for highly efficient synthesis of tetrahydroquinoline†
Abstract
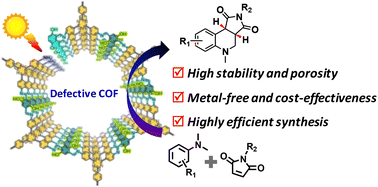
Cyclization of N,N-dimethylanilines with maleimides to obtain tetrahydroquinoline heterocyclic compounds is an essential reaction in industry but it is usually catalyzed by noble-metal catalysts under harsh conditions. High-performance, metal-free, low-cost, stable porous photocatalysts can provide an efficient pathway for the green synthesis of tetrahydroquinolines. Herein, we present a unique imine-based covalent organic framework, COF-HNU30-10, with good stability and a high density of structural defects for the selective cyclization of N,N-dimethylanilines with maleimides. Experiments show that various tetrahydroquinolines can be quantitatively synthesized (yield > 99.9%) using COF-HNU30-10 as a catalyst under visible-light irradiation. Such a yield is significantly higher than that (yield of 49%) obtained using a non-defective COFHNU30-0 and even higher than that obtained with Pt and Ru-based heterogeneous catalysts. A mechanistic study reveals that the introduction of defects in the framework facilitates the charge transfer and separation states, thus resulting in an enhancement of photocatalytic activity. Moreover, COF-HNU30-10 achieved a benchmark balance between the photocatalytic performance, stability and cost-effectiveness for the quantitative synthesis of tetrahydroquinolines.
- This article is part of the themed collection: Organic Chemistry in Green Chemistry: Key Highlights


 Please wait while we load your content...
Please wait while we load your content...