Biotransamination with racemic amines as amine donors: kill three birds with one stone through a dual-enzyme cascade†
Abstract
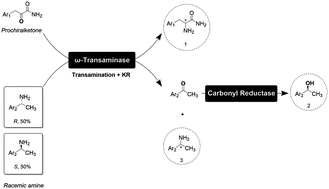
The obstacles in the widespread application of ω-transaminases for the production of chiral amines is inefficient equilibrium displacement and high costs of by-product removal. Hence, our study skillfully designed a two-enzyme cascade methodology that eliminates the requirement of costly NAD(P)H resources. By incorporating a suitable carbonyl reductase within an optimal pH range coinciding with the ω-transaminase to facilitate transamination, this innovative process makes it possible to remove coproducts via asymmetric reduction with the assistance of carbonyl reductase. Two chiral compounds are obtained by the addition of the desired chiral amines. The carbonyl reductase has three distinct functions: providing extra chiral alcohols and amines, removing coproducts from the reaction system, and shifting the equilibrium towards the product formation side. The establishment of this cascade can also serve as a blueprint for subsequent enzymatic processes involving internal thermal equilibria.


 Please wait while we load your content...
Please wait while we load your content...