A visible-light-promoted metal-free approach for N–H insertions by using donor/donor diazo precursors†
Abstract
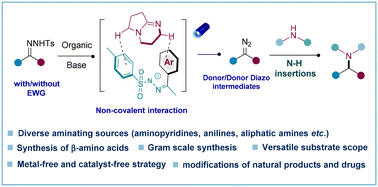
The metal-catalyzed carbenoid-based N–H insertions of α-stabilized diazo compounds and relative precursors serve as robust strategies for the formation of C–N bonds. However, carbenoid-based N–H insertions with aminopyridines are usually challenging due to the undesired coordination effect between the pyridine and the metal. Moreover, the coupling of donor/donor diazo compounds with amines remains challenging due to the instability and unavailability of donor/donor diazo compounds. Considering all these existing challenges, herein, a metal-free strategy is reported to achieve N–H insertions via coupling donor/donor diazo precursors (N-tosylhydrazones) with a plethora of amines including aminopyridines, anilines, aliphatic amines, and other nucleophiles such as imidazoles, pyrroles, and indoles. Expediently, β-amino esters are also afforded by using this mild strategy. The utility of this protocol is further demonstrated by the gram-scale synthesis and modifications of pharmaceuticals. Therefore, it is very clear that this metal-free approach uniquely tolerates a wide range of nucleophiles and opens a straightforward synthetic route to synthesize diverse value-added amines.
- This article is part of the themed collection: Organic Chemistry in Green Chemistry: Key Highlights


 Please wait while we load your content...
Please wait while we load your content...