Se-methylselenocysteine ameliorates mitochondrial function by targeting both mitophagy and autophagy in the mouse model of Alzheimer's disease†
Abstract
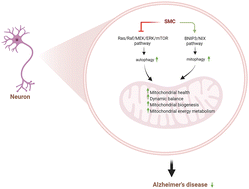
Background: Alzheimer's disease (AD) exerts tremendous pressure on families and society due to its unknown etiology and lack of effective treatment options. Our previous study had shown that Se-methylselenocysteine (SMC) improved the cognition and synaptic plasticity of triple-transgenic AD (3 × Tg-AD) mice and alleviated the related pathological indicators. We are dedicated to investigating the therapeutic effects and molecular mechanisms of SMC on mitochondrial function in 3 × Tg-AD mice. Methods: Transmission electron microscopy (TEM), western blotting (WB), mitochondrial membrane potential (ΔΨm), mitochondrial swelling test, and mitochondrial oxygen consumption test were used to evaluate the mitochondrial morphology and function. Mitophagy flux and autophagy flux were assessed with immunofluorescence, TEM and WB. The Morris water maze test was applied to detect the behavioral ability of mice. Results: The destroyed mitochondrial morphology and function were repaired by SMC through ameliorating mitochondrial energy metabolism, mitochondrial biogenesis and mitochondrial fusion/fission balance in 3 × Tg-AD mice. In addition, SMC ameliorated mitochondria by activating mitophagy flux via the BNIP3/NIX pathway and triggering autophagy flux by suppressing the Ras/Raf/MEK/ERK/mTOR pathway. SMC remarkably increased the cognitive ability of AD mice. Conclusions: This research indicated that SMC might exert its therapeutic effect by protecting mitochondria in 3 × Tg-AD mice.


 Please wait while we load your content...
Please wait while we load your content...