Degradation of benzotriazole and benzothiazole with the UV-activated peracetic acid process: performance, mechanism and transformation pathway†
Abstract
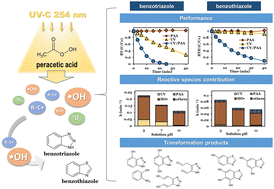
Benzotriazole (BT) and benzothiazole (BTH) are two high-production chemicals that are recalcitrant and have been commonly detected in aquatic environments worldwide, posing potential threats to ecosystems. The main purpose of this study was to utilize the UV-activated peracetic acid (UV/PAA) process to degrade BT and BTH in aqueous environments and explore the reaction kinetics and mechanisms involved. Both BT and BTH were efficiently degraded by UV/PAA, with pseudo first-order rate constants (k) of 0.155 and 0.059 min−1, respectively. The k values for BT and BTH decreased with increasing solution pH (3.0–11.0) and initial compound concentrations (0.04–0.24 mM), while k exhibited a positive and proportional relationship with the PAA dose (5–30 mg L−1). The degradation of BT and BTH via the UV/PAA reaction was mainly attributed to ·OH attack; UV photolysis, carbon-centered radicals (R–C·) and PAA oxidation also made contributions. The contributions of 1O2 to BT and BTH degradation were negligible despite the formation of 1O2 in the UV/PAA system. The effects of coexisting water components (dissolved organic matter, HCO3−, Cl− and NO3−) and the impact of actual wastewater matrix on BT and BTH degradation were also investigated. During the UV/PAA reaction, BT was degraded into products through pathways involving i) hydroxylation, ii) triazole ring opening and iii) benzene ring opening, while BTH underwent a continuous hydroxylation reaction. The results from a toxicity assessment indicated that BT and BTH were degraded and decomposed into less toxic products with the UV/PAA process, demonstrating the potential of the UV/PAA reaction for BT and BTH removal from aqueous environments.


 Please wait while we load your content...
Please wait while we load your content...